COVID-19 Rapid Antigen Testing

Warner & Webster is actively monitoring the availability of several Rapid Antingen Testing products in Australia, as the arrival of the Omicron Variant in the lead up to the Christmas-New Year period has significantly increased demand for these products.
We are working closely with suppliers to fill orders as quickly as possible, however the rapid surge in demand has meant that certain products are no longer available.
Warner & Webster are not accepting backorders at this time, and we apologise for any inconvienience this may cause.
Please note: Due to TGA restrictions, we are unable to supply the products below direct to consumers.
Click here for Rapid Antigen Self-Tests approved for use by the general public.
The COVID-19 Rapid Antigen Test is not a substitute for a PCR test, Rapid Antigen testing is only for screening purposes and you should rely on a PCR test if unwell at all.
This test is only a screening aid in detecting antigen from the SARS-CoV-2 virus in individuals suspected of COVID-19.
This product is strictly intended for professional use in laboratory and Point of Care environment.
What is the difference between nasal and nasypharyngeal tests?
The COVID-19 Rapid Antigen Test Nasal provides rapid results for fast decision making at the Point of Care. Nasal sampling is less invasive, which can reduce patient discomfort, and minimize the duration of the procedure as well as the risk of sneezing or coughing. Nasal swab samples may be self-collected by patients under supervision, offering further protection for healthcare professionals.
Rapid Antigen tests do not replace PCR tests, you should always consult your medical professional and get a PCR test if unwell.
The value of rapid testing is that they can detect an active infection before symptoms become apparent, especially when used regularly as part of a testing regime, while the patient is shedding high levels of the virus and therefore has a high risk of transmitting it to others.
Rapid Antigen tests do not replace PCR tests, you should always consult your medical professional and get a PCR test if unwell.
Can I purchase the test?
The tests can be supplied for use by specified health practitioners at the point of care to the following:
- Registered Medical Practitioners or Paramedics, or an organisation, business or institution that employs or engages a registered Medical Practitioner or Paramedic to perform or oversee performance of the test. The tests can only to be used to test employees or contractors of the organisation, business or institution, or a patient under the direct care of the Medical Practitioner or the Paramedic.
- Residential care (disability and rehabilitation facilities) and aged care facilities that employ or engage Health Practitioners (as defined by the Therapeutic Goods Act 1989) to conduct or perform the test. If the residential care or aged care facilities provide care in the home this condition would also allow for performance of the test to be conducted by a Health Practitioner or Paramedic. The tests can only be used to test residents, staff of, or visitors to, the residential care or aged care facility, or clients and staff of the home care service provider.
- Organisations, businesses, or institutions that employ or engage health practitioners or paramedics to conduct or oversee performance of the tests. For example, rapid antigen tests are being used in the mining sector consistent with these conditions. The tests can only be used to test staff or students of the organisation, business or institution, or a person who is a patient of a registered Dental Practitioner who requires an emergency dental procedure.
The tests can also be supplied to accredited laboratories and to Commonwealth, state or territory government departments, in cooperation with their relevant health departments.
I live in South Australia or Western Australia. Can I purchase Rapid Antigen Tests?
In South Australia, Point of care antigen based or nucleic acid based tests for detecting or diagnosing COVID-19 may be used by:
- a person providing pathology services within SA Pathology; or
- a person providing public health services within the Department of Health and Wellbeing; or
- NATA accredited pathology labs or similar testing services that have been approved by the SA Chief Public Health Officer.
In Western Australia, the Chief Health Officer has revoked the directions prohibiting the use of Rapid Antigen Tests as of 10 January 2022.
Panbio Testing Procedure
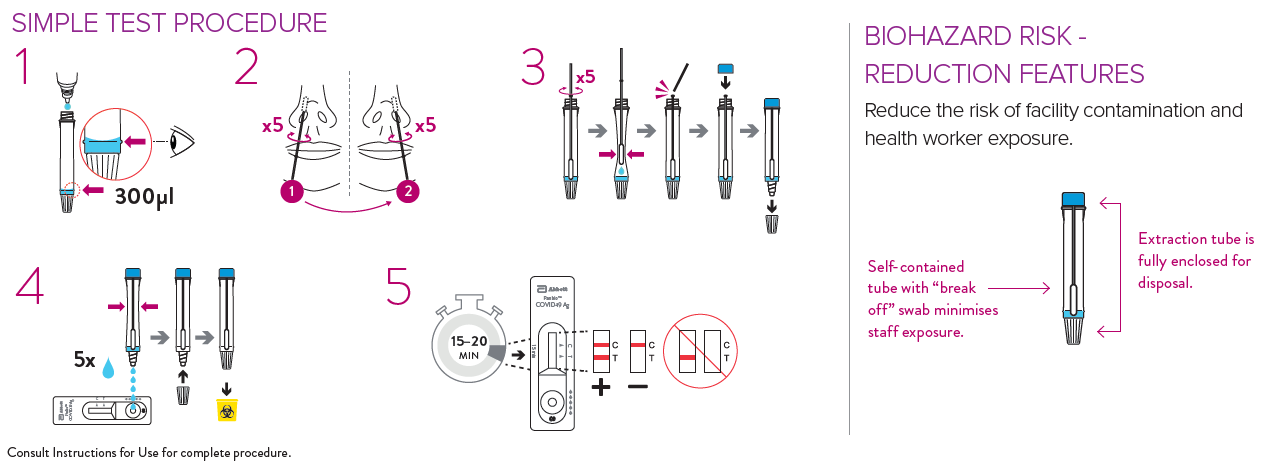
Panbio Training Videos
PanbioTM COVID-19 Ag Live Action Demo Video
Onsite Testing Procedure

OnSite training Video
What do I need to do to purchase the rapid antigen tests?
- Please make sure you watch the above training video before purchase and refer to it any time you require
- Complete the Customer Authorisation Form for the test you intend to purchase
- Wait for confirmation from Warner & Webster that your account has been setup to purchase your Rapid Antigen Test
- All purchases must be made on a Warner & Webster 30-day account with an associated medical practitioner
- Our New Account Application form can be found here if required
- Call our Customer Service team on 1300 556 917 or place your order through our Web Self-Service System.
- After purchasing this product, you will need to attend webinar training provided by the manufacturer before you begin testing.
Be aware, we will need to pass your contact details onto the manufacturer so they can provide this training. - Further information on COVID-19 rapid antigen testing in general is avaliable from the TGA at the links below
Which products are available?
If you are after 1,000 or more tests please give us a call on 1300 556 917
| Product Code | Product Description | Test Type | UoM | Brand |
| 225139 | COVID-19 Rapid Antigen Test Panbio 25s | Nasopharyngeal | Box/25 | Abbott |
| 225167 | COVID-19 Rapid Antigent Test OnSite 20s | Nasopharyngeal | Box/20 | OnSite |
Supporting Documentation
General Information from the Therapeutic Goods Administration
Abbott Panbio Tests
- Panbio COVID-19 Antigen Test Brochure
- Panbio COVID-19 Antigen Test Instructions for Use
- Panbio COVID-19 Antigen Test Quick Reference Guide
- Abbott Panbio Rapid Antigen Test Customer Authorisation Form (all jurisdictions exluding SA)
- Abbott Panbio Rapid Antigen Test Customer Authorisation Form (SA customers only)
- ARTG Certificate
OnSite Tests
- OnSite Antigen Test Brochure
- OnSite Antigen Test Instructions for Use
- Onsite Antigen Test Training Slides
- Establishing Your OnSite Screening Strategy
- Rapid Antigen Testing Flowchart
- Rapid Antigen Testing FAQs
- Record Keeping Template
- OnSite Rapid Antigen Test Customer Authorisation Form (not for sale to SA customers)
- ARTG Certificate
 1300 556 917
1300 556 917