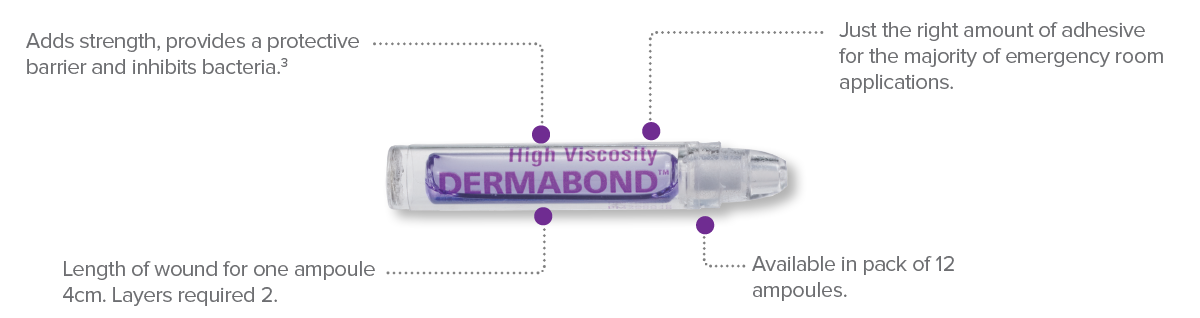
Dermabond
Meet the DERMABOND® Family
THE WORLD’S MOST TRUSTED WOUND CLOSURE PRODUCTS
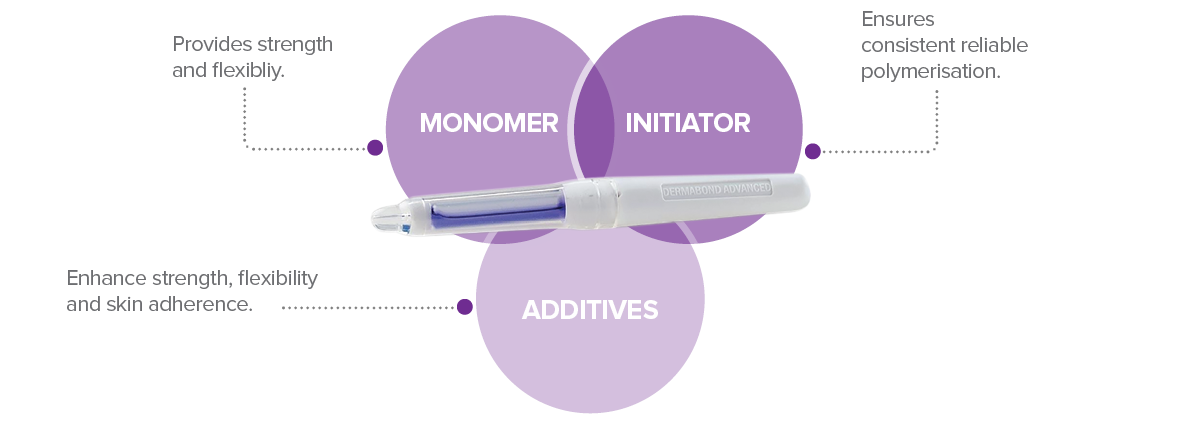
DERMABOND® is a precisely balanced formulation unlike any other TSA on the market
Our proprietary formulation is a precisely balanced combination of three carefully sourced components. There is no other brand with the same formula.
Topical skin adhesives (TSAs) are an integral part of a successful clinical outcome.
When deciding which TSA to use, clinical study information on closure strength, microbial protection, patient comfort, and cosmesis allows healthcare practitioners to evaluate which product will provide the greatest benefits for their patients.
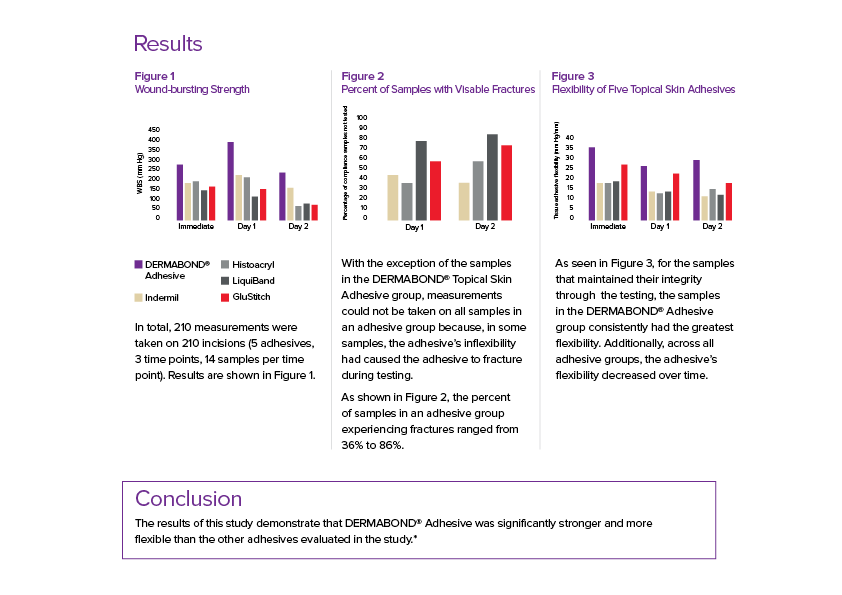
DERMABOND® Skin Adhesives is backed by an extensive body of published literature that has been shown to provide superior strength versus other commercially available TSA’s and also has benefits that enhance patient comfort and cosmetic outcomes.
Fortify your skin closure in 3 ways and achieve improved patient outcomes
Strengthens
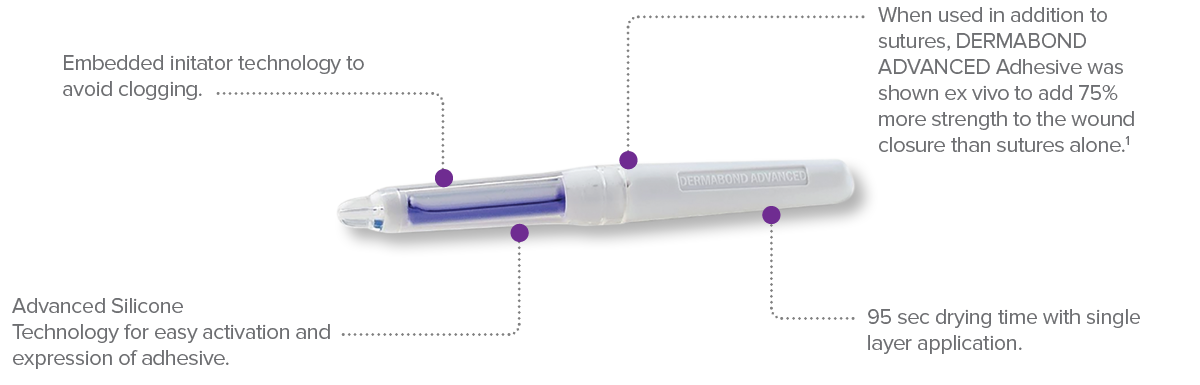
- When used in addition to sutures, DERMABOND Topical Skin Adhesive was shown ex vivo to add 75% more strength to the wound closure than sutures alone.
Inhibits Bacteria
- DERMABOND Topical Skin Adhesive demonstrated in vitro inhibition of gram positive bacteria (MRSA and MRSE) and gram negative bacteria (E.Coli) for 48 hours± 2 Provides a Microbial Barrier
Provides a Microbial Barrier
- DERMABOND Topical Skin Adhesive provides a flexible microbial barrier with >99% protection in vitro for at least 72 hours against organisms commonly responsible for SSIs2
DERMABOND® Mini Topical Skin Adhesive
A protective barrier that adds strength and inhibits bacteria
- Provides a microbial barrier with 99% protection in vitro for 72 hours against organisms commonly responsible for SSIs2
- Demonstrates inhibition of gram-positive bacteria (MRSA and MRSE) and gram-negative bacteria (E coli) in vitro
Unique formulation with additional value
- Addresses cost and convenience concerns by eliminating the need for return visits to remove sutures
- Offers fast closure of small incisions and lacerations
- Provides patient comfort by providing flexible closure without the pain or anxiety caused by needles
Click here to learn more about Dermabond Mini
DERMABOND® ADVANCED™ Topical Skin Adhesive
Designed for easy use and application
Patient Comfort & Satisfaction
- In a prospective randomised study of minimally invasive vein harvest closure in 106 patients surgeries, patient satisfaction, colour and visibility of scar was significantly better with Dermabond® Adhesive at 6 weeks
- In a retrospective study of bilateral mammoplasties in 670 patients, patient comfort and satisfaction were also superior with DERMABOND® Adhesive
- Patients can shower immediately as directed by their physician
Reduced Incidence of SSIs
- After CABG surgery, the incidence of SSIs was reduced by 57% for wounds closed with DERMABOND® Adhesive in addition to sutures, compared to wounds closed with sutures alone
Decreased Length of Stay
- The use of DERMABOND® Adhesive in CABG surgery was associated with allowing patients to return home 4 days earlier (9 days stay, instead of 13)
DERMABOND® PRINEO® Skin Closure System
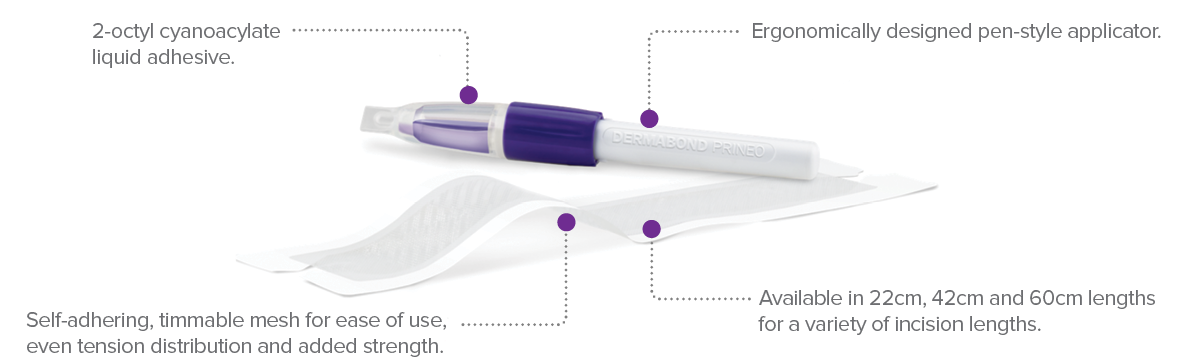
A combination of 2-octyl cyanoacrylate (2-OCA) and a self-adhering mesh
Protection
- Provides microbial-barrier protection 99% effective in vitro for 72 hours against organisms commonly responsible for surgical site infection (SSI)2
- Demonstrates in vitro inhibition of bacteria (Methicillin-resistant Staphylococcus aureus, Methicillin-resistant Staphylococcus epidermidis, Escherichia coli)
Strength
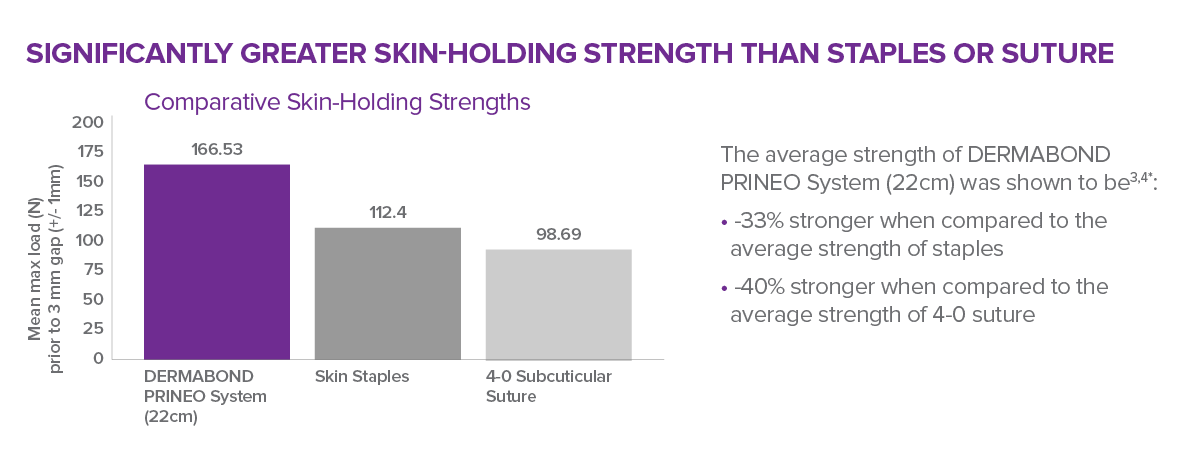
- Significantly greater skin holding strength than skin staples or subcuticular suture
- Redistributes tension away from the wound to the surrounding healthy surface area
Patient Satisfaction
- Patient may be able to shower immediately after the procedure, if directed by the healthcare professional
- At the time of removal, DERMABOND® PRINEO® System is associated with less pain than other wound closure devices
- No post-surgical dressings may mean easier self-care and greater self-confidence for patients
DERMABOND® PRINEO® family continues to grow, offering a closure solution for more surgical wounds - (22cm, 42cm and 60cm)
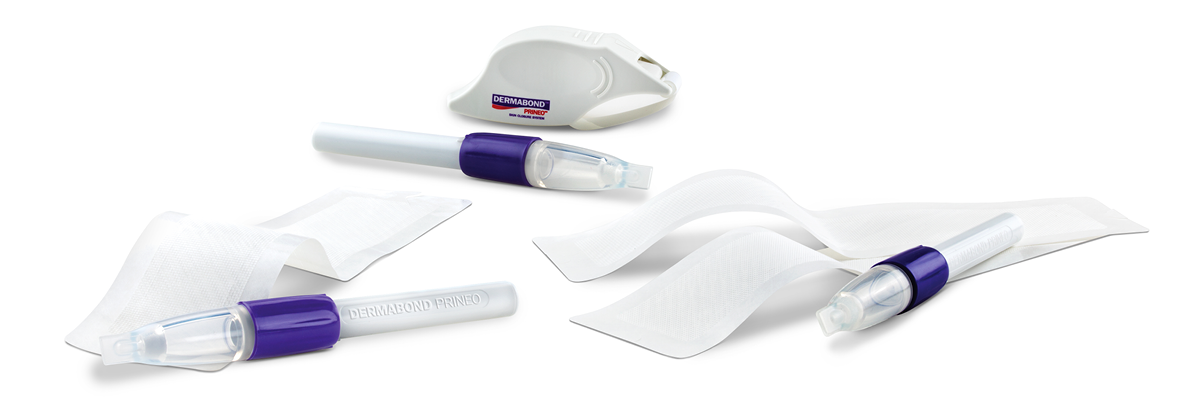
DERMABOND PRINEO System combines the proven strength, flexibility, and antimicrobial protection of DERMABOND ADVANCED® Topical Skin Adhesive with the added support and security of a self-adhering mesh to further facilitate both wound-edge approximation and an optimal healing environment4.
Shown to provide statistically significant greater skin-holding strength than skin staples or subcuticular sutures.
 1300 556 917
1300 556 917